Multiple Hydrate or Freeze-Out Points
Lili Lyddon
January 23, 2007
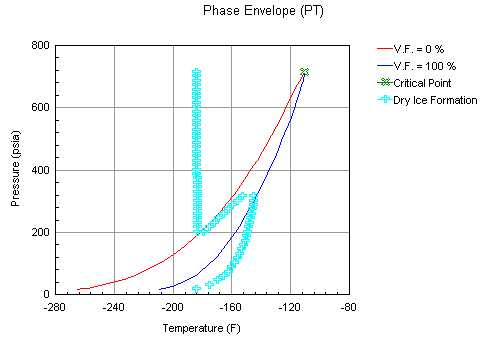
Depending on composition and pressure, multiple hydrate, water freeze out, or CO2 freeze out temperatures are possible. ProMax calculates up to three of these points. When only a single point is present, the solid is stable at all temperatures below that point. When three points are calculated, the solid is stable at temperatures between the Mid and High Temperature Hydrate/Freeze Points and below the Low Temperature Hydrate/Freeze Point, but is not stable between the Low and Mid Temperature Hydrate/Freeze Points. The phase envelope analysis provides a graphical view of this behavior. For an example of multiple CO2 freeze points, consider the following composition at a pressure of 250 psig: Nitrogen = 1.45 mole%, Carbon Dioxide = 1.20, Methane = 96.30, Ethane = 1.00, and Propane = 0.05. The plot of the solid CO2 (dry ice) curve on the Phase Envelope provides a visual representation of this behavior:
Authored by Lili Lyddon (BR&E Technical Support and Help Author)